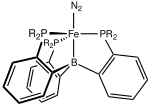
Transition metal dinitrogen complex
[8] From the late 1960s, a variety of transition metal-dinitrogen complexes were made including those with iron,[9] molybdenum[10] and vanadium[11] as metal centers.
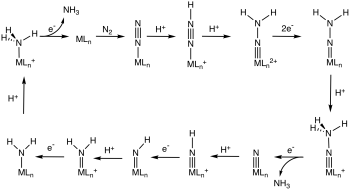
Biological nitrogen fixation probably occurs via the binding of N2 to those metal centers in the enzyme nitrogenase, followed by a series of steps that involve electron transfer and protonation.
Based on whether the N2 molecules are shared by two more metal centers, the complexes can be classified into mononuclear and bridging.
Based on the geometric relationship between the N2 molecule and the metal center, the complexes can be classified into end-on or side-on modes.
In the end-on bonding modes of transition metal-dinitrogen complexes, the N-N vector can be considered in line with the metal ion center, whereas in the side-on modes, the metal-ligand bond is known to be perpendicular to the N-N vector.
Transition metal-dinitrogen complexes can contain more than one N2 as "end-on" ligands, such as mer-[Mo(N2)3(PPrn2Ph)3], which has octahedral geometry.
N2 also serves as a bridging ligand with "end-on" bonding to two metal centers, as illustrated by {[Ru(NH3)5]2(μ-N2)}4+.
[18] Hasanayn and co-workers have shown that the Lewis structures of end-on bridging complexes can be assigned based on π-molecular-orbital occupancy, in analogy with simple tetratomic organic molecules.
For example the cores of N2-bridged complexes with 8, 10, or 12 π-electrons can generally be formulated, respectively, as M≡N-N≡M, M=N=N=M, and M-N≡N-M, in analogy with the 8-, 10-, and 12-π-electron organic molecules HC≡C-C≡CH, O=C=C=O, and F-C≡C-F.[19] In comparison with their end-on counterpart, the mononuclear side-on dinitrogen complexes are usually higher in energy and the examples of them are rare.
Fomitchev and Coppens has reported the first crystallographic evidence for side-on coordination of N2 to a single metal center in a photoinduced metastable state.