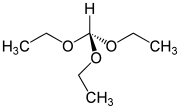
Triethyl orthoformate
Triethyl orthoformate is an organic compound with the formula HC(OC2H5)3.
This colorless volatile liquid, the ortho ester of formic acid, is commercially available.
[1] It may also be prepared from the reaction of sodium ethoxide, formed in-situ from sodium and absolute ethanol, and chloroform:[2] Triethyl orthoformate is used in the Bodroux-Chichibabin aldehyde synthesis, for example:[3] In coordination chemistry, it is used to convert metal aquo complexes to the corresponding ethanol complexes:[4] Triethyl orthoformate (TEOF) is an excellent reagent for converting compatible carboxylic acids to ethyl esters.
Such carboxylic acids, refluxed neat in excess TEOF until low-boilers cease evolution, are quantitatively converted to the ethyl esters, without need for extraneous catalysis.
[5] Alternatively, added to ordinary esterifications using catalytic acid and ethanol, TEOF helps drive esterification to completion by converting the byproduct water formed to ethanol and ethyl formate.