Trimeric autotransporter adhesin
In molecular biology, trimeric autotransporter adhesins (TAAs), are proteins found on the outer membrane of Gram-negative bacteria.
The head domain, once assembled, then adheres to an element of the host extracellular matrix, for example, collagen, fibronectin, etc.
In addition, all membrane anchor domains are of the left-handed parallel beta-roll type.
The function of the ESPR is to aid inner membrane translocation[5] by acting as a temporary tether.
[6] The ESPR can be divided into individual regions, they are as follows: N1 (charged), H1 (hydrophobic), N2, H2 and C (cleavage site) domains.
[7] Function: There are several roles that the Extended Signal Peptide Region is thought to hold.
The head domain is connected to the stalk by a short, highly conserved sequence, which is often called the neck, or occasionally named the connector.
[11] Each domain helps the head to bind to a different component of the extracellular matrix.
The Trp ring obtains its name from the high levels of tryptophan found in the C-terminal part of the Head domain.
[16] These work by stabilising the transition between the coiled-coil and the beta-meander where the head meets the neck or stalk.
It has an all-beta structure, whereby the two pairs of antiparallel beta sheets are connected by a diagonally running extended beta-sheet.
The sheets then further fold to form a beta prism in which each wall is composed of a complete set of five beta-strands.
Hence, the stalk domains can be considered alpha helical coiled-coils that deviate from the standard model due to their unusual properties.
Then, once the knobs are packed into cavities, the three helices are wound in register around each other, so all of the residues in certain positions are at the same height.
[16] Function: Their role is to act as spacers by moving the head domains away from the bacterial cell surface and toward the extracellular matrix of the host.
[23] All Trimeric Autotransporter Adhesins are crucial virulence factors that cause serious disease in humans.
[8] Homotrimerisation is a process whereby three of the same subunits, associate to make a complex of three identical YadA proteins.
The head region of YadA is composed of beta-helices further folded to create a nine-coiled left-handed parallel beta-roll (LPBR).
The NadA protein is found in a species of Gram-negative bacteria called Neisseria meningitidis, which causes sepsis and meningitis in humans.
[27] Studies have shown that the globular N-terminal head domain of NadA is vital for adhesion.
[25] The Hia protein is a TAA found on the outer membrane of the bacterium Haemophilus influenzae.
[30] As the name suggests, it holds three beta sheets arranged in a triangular prism and contains internal symmetry.
Bartonella henselae is the causative agent of cat scratch disease, a normally harmless disease, but, in people with a weakened immune system, such as those undergoing chemotherapy or fighting AIDS, it is more serious as it can lead to bacillary angiomatosis.
BadA also contains a neck domain, an extended coil-coil stalk, and beta-barrel C terminal membrane anchor.
Then the Trimeric Autotransporter Adhesin must adhere to the layer of cells found on the internal surface, the epithelial cells, in the intestine by using its head to bind to proteins found in the extracellular matrix such as collagen, laminin, and fibronectin.
[8] It is important that these outer-membrane adhesins make physical contact with the receptors found on the host cell.
TAAs are a type of microbial surface components recognizing adhesive matrix molecules (MSCRAMMs).
Gram-negative bacteria must secrete adhesins, since they have an outer membrane that makes it hard for them to stick to and infect the host.
[3] The type V secretion system is described as non-fimbrious, meaning that the bacterial cells do not use long physical appendages named pili to attach to one another.
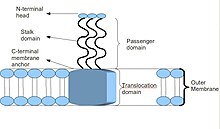
Schematic diagram of the basic Trimeric Autotransporter Adhesin structure
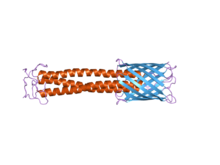
The C-terminal membrane anchor domain can clearly be seen on the right in blue. The stalk domain can be seen in red.

The protein domain arrangement of the Trimeric Autotransporter Adhesin, BadA [ 1 ] This figure shows the head, stalk and anchor domains. It shows the YadA-like head in grey. The stalk contains repeats coloured in green and the membrane anchor in red. The sequence below shows colouring according to domain arrangement and protease cleavage sites red (trypsin) and blue (chymotrypsin). (Figure used from open access journal, in the public domain, Public Library of Science (PLoS) Pathogen

Comparison of Head domains in different Trimeric Autotransporter Adhesins [ 10 ] (Figure used from open access journal, in the public domain, Public Library of Science (PLoS) Pathogen)
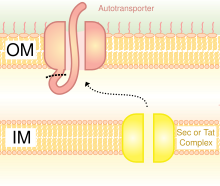
A schematic diagram illustrating the Trimeric Autotransporter Adhesins in Type V Secretion System.