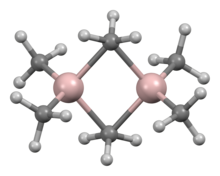
Trimethylaluminium
[6]TMA is prepared via a two-step process that can be summarized as follows: Starting with the invention of Ziegler-Natta catalysis, organoaluminium compounds have a prominent role in the production of polyolefins, such as polyethylene and polypropylene.
TMA is the preferred precursor for metalorganic vapour phase epitaxy (MOVPE) of aluminium-containing compound semiconductors, such as AlAs, AlN, AlP, AlSb, AlGaAs, AlInGaAs, AlInGaP, AlGaN, AlInGaN, AlInGaNP, etc.
For example, dimethylamine gives the dialuminium diamide dimer:[7] TMA reacts with many metal halides to install alkyl groups.
Tebbe's reagent, which is used for the methylenation of esters and ketones, is prepared from TMA and titanocene dichloride.
[9] In combination with 20 to 100 mol % Cp2ZrCl2 (zirconocene dichloride), the (CH3)2Al-CH3 adds "across" alkynes to give vinyl aluminium species that are useful in organic synthesis in a reaction known as carboalumination.
[12] The NASA ATREX mission (Anomalous Transport Rocket Experiment) employed the white smoke that TMA forms on air contact to study the high altitude jet stream.