Tumor M2-PK
Increased Tumor M2-PK values can sometimes also occur in severe inflammatory diseases, which must be excluded by differential diagnosis.
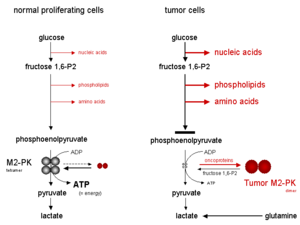
When M2-PK is mainly in the highly active tetrameric form, which is the case in most normal cells, glucose is mostly converted to lactate, with the attendant production of energy.
In contrast, the dimeric form of M2-PK has a low affinity for phosphoenolpyruvate, being nearly inactive at physiological PEP concentrations.
As a consequence of the key position of pyruvate kinase within glycolysis, the tetramer : dimer ratio of M2-PK determines whether glucose carbons are converted to pyruvate and lactate, along with the production of energy (tetrameric form), or channelled into synthetic processes (dimeric form).
Thereafter, the cycle of oscillation starts again when the fructose 1,6-P2 levels reach a certain upper threshold value which induces the tetramerization of M2-PK.
When M2-PK is mainly in the less active dimeric form, energy is produced by the degradation of the amino acid glutamine to aspartate, pyruvate and lactate, which is termed glutaminolysis.
In tumor cells the increased rate of lactate production in the presence of oxygen is termed the Warburg effect.
For the first time pyruvate kinase M2 enzyme was reported with two missense mutations, H391Y and K422R, found in cells from Bloom syndrome patients, prone to develop cancer.
H391Y showed a 6-fold increase in affinity for its substrate phosphoenolpyruvate and behaved like a non-allosteric protein with compromised cooperative binding.
[7] The co-expression of homotetrameric wild type and mutant PKM2 in the cellular milieu resulting in the interaction between the two at the monomer level was substantiated further by in vitro experiments.