Urokinase
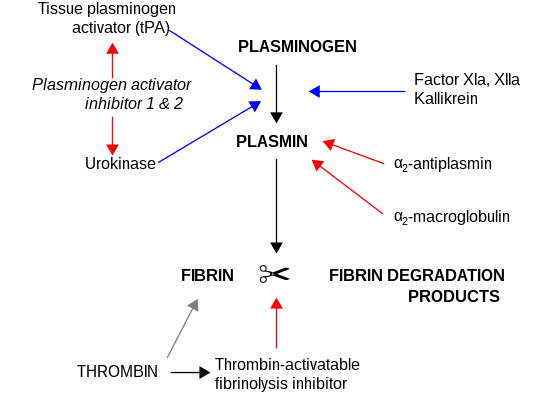
The primary physiological substrate of this enzyme is plasminogen, which is an inactive form (zymogen) of the serine protease plasmin.
Activation of plasmin triggers a proteolytic cascade that, depending on the physiological environment, participates in thrombolysis or extracellular matrix degradation.
The PLAU gene encodes a serine protease (EC 3.4.21.73) involved in degradation of the extracellular matrix and possibly tumor cell migration and proliferation.
[9] In comparison to the mammalian system, zebrafish (Danio rerio) contains two orthologs of urokinase which have been characterised as zfuPA-a and zfuPA-b.
[10] Elevated expression levels of urokinase and several other components of the plasminogen activation system are found to be correlated with tumor malignancy.
As of December 7, 2012, Mesupron (upamostat), a small molecule serine protease inhibitor developed by the WILEX pharmaceutical company, has completed phase II trials.
[17] Mesupron appears to be safe when combined with chemotherapeutic drug Capecitabine for the progression-free survival in human breast cancer.
Urokinase is also used clinically as a thrombolytic agent in the treatment of severe or massive deep venous thrombosis, peripheral arterial occlusive disease, pulmonary embolism, acute myocardial infarction (AMI, heart attack), and occluded dialysis cannulas (catheter clearance).
Urokinase is marketed as Kinlytic (formerly Abbokinase) and competes with recombinant tissue plasminogen activator (e.g., alteplase) as a thrombolytic drug.
All plasminogen activators (urokinase, tPA) catalyze the production of plasmin, which in turn leads to the breakdown of the fibrin mesh structure in blood clots.
This makes urokinase less likely to break down such hemostatic clots that are essential for ongoing blood vessel repair throughout the body.
[23] The full text for this article is lost, and the only citation points to the abstract of a list of papers read at a conference in the same journal.