Angiogenesis
However, it is also a fundamental step in the transition of tumors from a benign state to a malignant one, leading to the use of angiogenesis inhibitors in the treatment of cancer.
As sprouts extend toward the source of the angiogenic stimulus, endothelial cells migrate in tandem, using adhesion molecules called integrins.
These cells begin laying collagen fibers into the core to provide an extracellular matrix for growth of the vessel lumen.
Chemical stimulation of angiogenesis is performed by various angiogenic proteins e.g. integrins and prostaglandins, including several growth factors e.g. VEGF, FGF.
In general, FGFs stimulate a variety of cellular functions by binding to cell surface FGF-receptors in the presence of heparin proteoglycans.
They stimulate the proliferation of fibroblasts and endothelial cells that give rise to angiogenesis and developing granulation tissue; both increase blood supply and fill up a wound space/cavity early in the wound-healing process.
Vascular endothelial growth factor (VEGF) has been demonstrated to be a major contributor to angiogenesis, increasing the number of capillaries in a given network.
[25] Upregulation of VEGF is a major component of the physiological response to exercise and its role in angiogenesis is suspected to be a possible treatment in vascular injuries.
[26][27][28][29] In vitro studies clearly demonstrate that VEGF is a potent stimulator of angiogenesis because, in the presence of this growth factor, plated endothelial cells will proliferate and migrate, eventually forming tube structures resembling capillaries.
The increase in receptor production means muscle contractions could cause upregulation of the signaling cascade relating to angiogenesis.
[31] These enzymes are highly regulated during the vessel formation process because destruction of the extracellular matrix would decrease the integrity of the microvasculature.
[35] Class 3 semaphorins (SEMA3s) regulate angiogenesis by modulating endothelial cell adhesion, migration, proliferation, survival and the recruitment of pericytes.
[38] Application of specific compounds that may inhibit or induce the creation of new blood vessels in the body may help combat such diseases.
Other diseases, such as age-related macular degeneration, may be created by a local expansion of blood vessels, interfering with normal physiological processes.
One of the first applications of pro-angiogenic methods in humans was a German trial using fibroblast growth factor 1 (FGF-1) for the treatment of coronary artery disease.
Difficulties include effective integration of the therapeutic genes into the genome of target cells, reducing the risk of an undesired immune response, potential toxicity, immunogenicity, inflammatory responses, and oncogenesis related to the viral vectors used in implanting genes and the sheer complexity of the genetic basis of angiogenesis.
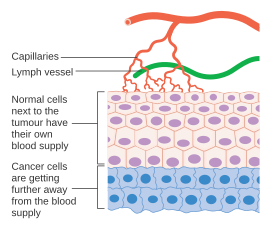
However, tumors need a dedicated blood supply to provide the oxygen and other essential nutrients they require in order to grow beyond a certain size (generally 1–2 mm3).
[46] Other clinicians believe angiogenesis really serves as a waste pathway, taking away the biological end products secreted by rapidly dividing cancer cells.
In either case, angiogenesis is a necessary and required step for transition from a small harmless cluster of cells, often said to be about the size of the metal ball at the end of a ball-point pen, to a large tumor.
[48] The mechanism of blood vessel formation by angiogenesis is initiated by the spontaneous dividing of tumor cells due to a mutation.
[49] Certain studies have indicated that vessels formed inside the tumor tissue are of higher irregularity and bigger in size, which is as well associated with poorer prognosis.
It is a potent, physiological process that underlies the natural manner in which our bodies respond to a diminution of blood supply to vital organs, namely neoangiogenesis: the production of new collateral vessels to overcome the ischemic insult.
Reproducible and credible successes in these early animal studies led to high enthusiasm that this new therapeutic approach could be rapidly translated to a clinical benefit for millions of patients in the Western world with these disorders.
A decade of clinical testing both gene- and protein-based therapies designed to stimulate angiogenesis in underperfused tissues and organs, however, has led from one disappointment to another.
Although all of these preclinical readouts, which offered great promise for the transition of angiogenesis therapy from animals to humans, were in one fashion or another, incorporated into early stage clinical trials, the FDA has, to date (2007), insisted that the primary endpoint for approval of an angiogenic agent must be an improvement in exercise performance of treated patients.
[52] These failures suggested that either these are the wrong molecular targets to induce neovascularization, that they can only be effectively used if formulated and administered correctly, or that their presentation in the context of the overall cellular microenvironment may play a vital role in their utility.
Anti-angiogenic drugs targeting the VEGF pathways are now used successfully to treat this type of macular degeneration Angiogenesis of vessels from the host body into an implanted tissue engineered constructs is essential.
Successful integration is often dependent on thorough vascularisation of the construct as it provides oxygen and nutrients and prevents necrosis in the central areas of the implant.
[55] The first report of angiogenesis can be traced back to the book A treatise on the blood, inflammation, and gun-shot wounds published in 1794, where Scottish anatomist John Hunter's research findings were compiled.
[10][56][57] Quantifying vasculature parameters such as microvascular density has various complications due to preferential staining or limited representation of tissues by histological sections.