Stereoisomerism
[1][2] This contrasts with structural isomers, which share the same molecular formula, but the bond connections or their order differs.
[3] Enantiomers, also known as optical isomers, are two stereoisomers that are related to each other by a reflection: they are mirror images of each other that are non-superposable.
Pure enantiomers also exhibit the phenomenon of optical activity and can be separated only with the use of a chiral agent.
In nature, only one enantiomer of most chiral biological compounds, such as amino acids (except glycine, which is achiral), is present.
A Fischer projection can be used to differentiate between L- and D- molecules Chirality (chemistry).
For instance, by definition, in a Fischer projection the penultimate carbon of D-sugars are depicted with hydrogen on the left and hydroxyl on the right.
The other refers to Optical rotation, when looking at the source of light, the rotation of the plane of polarization may be either to the right (dextrorotary — d-rotary, represented by (+), clockwise), or to the left (levorotary — l-rotary, represented by (−), counter-clockwise) depending on which stereoisomer is dominant.
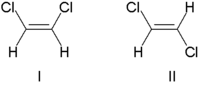
Due to occasional ambiguity, IUPAC adopted a more rigorous system wherein the substituents at each end of the double bond are assigned priority based on their atomic number.
The conformational inversion of substituted cyclohexanes is a very rapid process at room temperature, with a half-life of 0.00001 seconds.
[19] Le Bel-van't Hoff rule states that for a structure with n asymmetric carbon atoms, there is a maximum of 2n different stereoisomers possible.