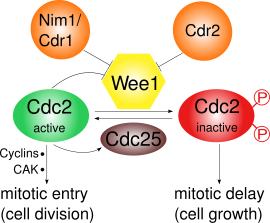
Wee1
[citation needed] Its name is derived from the Scottish dialect word wee, meaning small - its discoverer Paul Nurse was working at the University of Edinburgh in Scotland at the time of discovery.
[4] Following this data, Nurse, together with Pierre Thuriaux, analyzed fifty two Wee mutants undergoing mitosis – those lacking in Wee1 were comparatively smaller; their analysis led them to a model (later demonstrated to be true) where Wee1 is a dosage-dependent inhibitor of Cdc2, whose activity is required for a cell’s entry into M phase.
[5][3] As a result of these discoveries and its contributions to our understanding of cell cycle control, Nurse went on to win the 2001 Nobel Prize in Medicine or Physiology (shared also with Lee Hartwell and Tim Hunt).
[8] And while Wee1 and PKMYT1 both play critical roles in regulating entry into mitosis (i.e. working together to inhibit Cdk1 as it moves in / out of the cell nucleus), WEE1B was first discovered in Xenopus oocytes and is most active at the metaphase II exit point of meiosis prior to fertilization.
Kruppel-like factor 2 (KLF2) negatively regulates human WEE1, thus increasing sensitivity to DNA-damage induced apoptosis in cancer cells.
In some cases, studies have reported an observed overexpression of Wee1 in solid tumors / cancers like glioblastoma, hepatocellular carcinoma, melanoma, and more, as well as an association between elevated Wee1 expression and negative prognostic factors such as chemotherapy / radiotherapy resistance or other proliferation biomarkers; there are also accounts on an increased prevalence of upregulated Wee1 in instances there is also p53 loss of function.
Contrary to perhaps the typical rationale behind using inhibitor drugs to trigger cell cycle arrest, Wee1 inhibitors instead disrupt the S / G2 checkpoints to prematurely induce mitosis in cells; this mechanism thus promotes a phenomenon called “mitotic catastrophe” – increased DNA damage (insufficient repair or new events such as double-stranded breaks) that ultimately lead to apoptosis.
[27] At the cellular level, inhibiting Wee1 significantly reduces the presence of Tyr15, which consequently allows for Cdk1 kinase activity to build up and trigger entry into mitosis.
Adavosertib (AZD1775, MK1755), developed by AstraZeneca, is a small molecule Wee1 inhibitor, and was the first of its kind to reach clinical trials.
[28] Although in 2022, the company itself removed the drug from its pipeline, multiple trials testing adavosertib as both a monotherapy or in combination were conducted.
Results from randomized phase II trial FOCUS4-C demonstrated an around 2 month progression-free survival (PFS) improvement in those treated with adavosertib alone (versus the active monitoring control group);[29] meanwhile, another trial evaluating the efficacy of adavosertib in both patients with solid tumors and clear cell renal cell carcinoma observed no objective responses (with stable disease as the best response in 10/18 enrolled patients).
Phase I data for ZN-c3 (azenosertib, developed by Zentalis Therapeutics) shows early signs of clinical activity (2 partial responses and 5 stable disease out of 16 dosed patients) and tolerability;[33] however, several azenosertib trials were temporarily placed on clinical hold by the FDA between June and September 2024 after two patient deaths occurred.
Since Wee1 inhibits entry into mitosis, its absence will lead to division at a premature stage and sub-normal cell size.