Wallach rearrangement
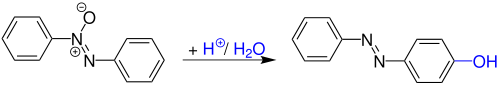
[1][2][3] The Wallach rearrangement is an organic reaction converting an aromatic azoxy compound with sulfuric acid or other strong acids to an azo compound with one arene ring substituted by a hydroxyl group in the aromatic para position.
In the first part of the reaction, two equivalents of acid tease the oxygen atom away from the azoxy group.
The resulting dicationic intermediate with an unusual R–N+=N+–R motif in this scheme has been observed by proton NMR in a system of fluoroantimonic acid and azoxybenzene at −50 °C.
The reaction mechanism for this reaction is not known with great precision despite experimental evidence: A mechanism not inconsistent with these findings is depicted below:[2] First, azoxybenzene undergoes protonation twice to afford a gitionic intermediate.
Water is eliminated to give the inferred symmetric intermediate, which is again gitionic and superelectrophilic.