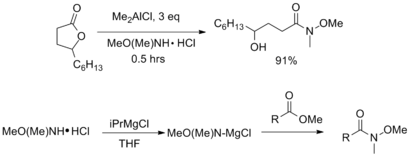
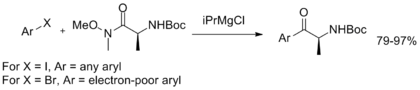Weinreb ketone synthesis
Their suggestion was that the tetrahedral intermediate (A below) formed as a result of nucleophilic addition by the organometallic reagent is stabilized by chelation from the methoxy group as shown.
[5] In addition to the original procedure shown above (which may have compatibility issues for sensitive substrates), Weinreb amides can be synthesized from a variety of acyl compounds.
The vast majority of these procedures utilize the commercially available salt N,O-dimethylhydroxylamine hydrochloride [MeO(Me)NH•HCl], which is typically easier to handle than the free amine.
[8] The standard conditions for the Weinreb–Nahm ketone synthesis are known to tolerate a wide variety of functional groups elsewhere in the molecule, including alpha-halogen substitution, N-protected amino acids, α-β unsaturation, silyl ethers, various lactams and lactones, sulfonates, sulfinates, and phosphonate esters.
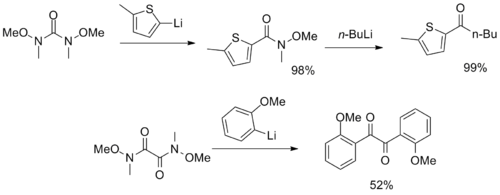
Lithiates and Grignard reagents are most commonly employed; examples involving aliphatic, vinyl, aryl, and alkynyl carbon nucleophiles have been reported.
However, with highly basic or sterically hindered nucleophiles, elimination of the methoxide moiety to release formaldehyde can occur as a significant side reaction.