Xenobiotic metabolism
The reactions in these pathways are of particular interest in medicine as part of drug metabolism and as a factor contributing to multidrug resistance in infectious diseases and cancer chemotherapy.
These pathways are also important in environmental science, with the xenobiotic metabolism of microorganisms determining whether a pollutant will be broken down during bioremediation, or persist in the environment.
That the exact compounds an organism is exposed to will be largely unpredictable, and may differ widely over time, is a major characteristic of xenobiotic toxic stress.
Polar compounds cannot diffuse across these cell membranes, and the uptake of useful molecules is mediated through transport proteins that specifically select substrates from the extracellular mixture.
However, the existence of a permeability barrier means that organisms were able to evolve detoxification systems that exploit the hydrophobicity common to membrane-permeable xenobiotics.
These enzyme complexes act to incorporate an atom of oxygen into nonactivated hydrocarbons, which can result in either the introduction of hydroxyl groups or N-, O- and S-dealkylation of substrates.
[5] The reaction mechanism of the P-450 oxidases proceeds through the reduction of cytochrome-bound oxygen and the generation of a highly-reactive oxyferryl species, according to the following scheme:[6]
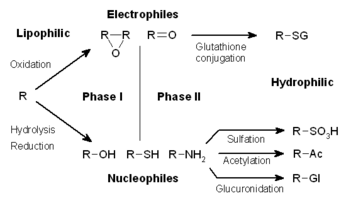
In subsequent phase II reactions, these activated xenobiotic metabolites are conjugated with charged species such as glutathione (GSH), sulfate, glycine, or glucuronic acid.
The addition of large anionic groups (such as GSH) detoxifies reactive electrophiles and produces more polar metabolites that cannot diffuse across membranes, and may, therefore, be actively transported.
Conjugates and their metabolites can be excreted from cells in phase III of their metabolism, with the anionic groups acting as affinity tags for a variety of membrane transporters of the multidrug resistance protein (MRP) family.
Studies on how people transform the substances that they ingest began in the mid-nineteenth century, with chemists discovering that organic chemicals such as benzaldehyde could be oxidized and conjugated to amino acids in the human body.
[12] This modern biochemical research resulted in the identification of glutathione S-transferases in 1961,[13] followed by the discovery of cytochrome P450s in 1962,[14] and the realization of their central role in xenobiotic metabolism in 1963.