Xylose isomerase
This enzyme belongs to the family of isomerases, specifically those intramolecular oxidoreductases interconverting aldoses and ketoses.
[3] The activity of D-xylose isomerase was first observed by Mitsuhashi and Lampen in 1953 in the bacterium Lactobacillus pentosus.
[5] In 1957, the D-xylose isomerase activity on D-glucose conversion to D-fructose was noted by Kooi and Marshall.
Hence D-xylose isomerase is used to produce these rare sugars which have very important applications in biology despite their low abundance.
[11] Xylose isomerase can be isolated from red Chinese rice wine, which contains the bacterium Lactobacillus xylosus.
[12] This bacterium was mistakenly classified as a L. plantarum, which normally grows on the sugar L-arabinose, and rarely grown on D-xylose.
[14] Its optimum growth pH is about 7.5 for the L. lactis, however strains such as the L.brevis xylose enzyme prefer a more alkaline environment.
[14] Thermal tests were also done by Kei Y. and Noritaka T. and the xylose isomerase was found to be thermally stable to about 60 degrees Celsius[14] Xylose isomerase has a structure that is based on eight alpha/beta barrels that create an active site holding two divalent magnesium ions.
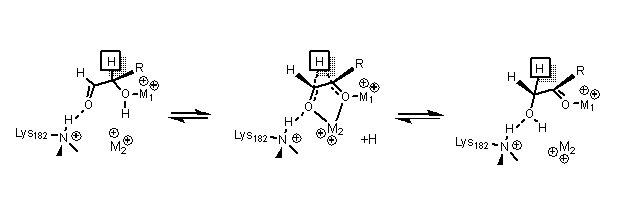
[16] In the isomerization of glucose, Histidine 53 is used to catalyze the proton transfer of O1 to O5; the diagram for the ring opening mechanism is shown below.
[17][16] The transition state consists of a high energy carbonium ion that is stabilized through all the metal interactions with the sugar substrate.
[23][25][22] This decrease in breath hydrogen excretion demonstrated in this study is a potential sign that fructose was absorbed much better.