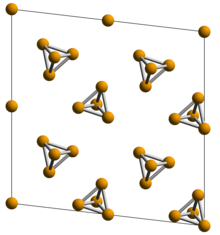
White phosphorus
[3] It glows greenish in the dark (when exposed to oxygen) and is highly flammable and pyrophoric (self-igniting) upon contact with air.
The odour of combustion of this form has a characteristic garlic odor, and samples are commonly coated with white "diphosphorus pentoxide", which consists of P4O10 tetrahedra with oxygen inserted between the phosphorus atoms and at their vertices.
[7] The β form of white phosphorus contains three slightly different P4 molecules, i.e. 18 different P-P bond lengths — between 2.1768(5) and 2.1920(5) Å.
It ignites spontaneously in air at about 50 °C (122 °F), and at much lower temperatures if finely divided (due to melting-point depression).
In the industrial process, phosphate rock is heated in an electric or fuel-fired furnace in the presence of carbon and silica.
[10] Most (83% in 1988) white phosphorus is used as a precursor to phosphoric acid, half of which is used for food or medical products where purity is important.
Chronic low-level exposure leads to tooth loss and phossy jaw which appears to be caused by the formation of amino bisphosphonates.
There are anecdotal reports of problems for beachcombers who may collect washed-up samples while unaware of their true nature.