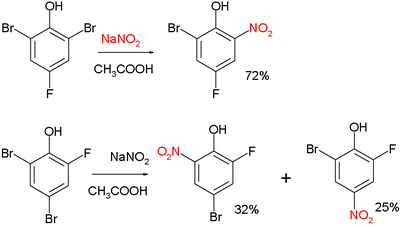
Zincke nitration
The Zincke nitration is a nitration reaction in which a bromine is replaced by a nitro group on an electron-rich aryl compound such as a phenol or cresol.
Typical reagents are nitrous acid or sodium nitrite.
The reaction is a manifestation of nucleophilic aromatic substitution and is named after Theodor Zincke, who first reported it in 1900.
[1][2] Two examples:[3] and:[4] The Zincke nitration should not be confused with the Zincke–Suhl reaction or the Zincke reaction.