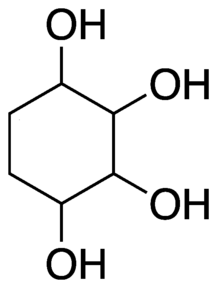
1,2,3,4-Cyclohexanetetrol
[1] The possible isomers are: Synthesis of 1,2,3,4-cyclohexanetretrols was first reported in 1933 by Pierre Bedos and Adrien Ruyer, by hydrolysis of 1,2;3,4-diepoxy-cyclohexane.
[6] Posternak and Reymond observed in 1953 that the 1,3/2,4 isomer (D and L forms) is not attacked by a certain strain of A. suboxydans, whereas all the others were metabolized with consumption of 1 atom of oxygen (possibly by formation of a ketone-triol), except the 1,2/3,4 isomer (D and L) that consumed 2 atoms.
[7] In 1955, Posternak and Reymond studied the oxydation of the 1,4/2,3 isomer (dihydro-conduritol) by Acetobacter suboxydans, producing a trihydroxyketone.
[3] In 2007, Peter Valente and others described the preparation of achiral 1,4/2,3-cyclohexanetetrol (toxocarol) from 2,3-dioxabicyclo[2.2.2]oct-5-ene, a cyclohexene with a peroxide bridge (–O–O–) replacing hydrogens in carbons 3 and 6.
The authors found that, by reversing the order of the two steps, they could obtain 1,4/2,3 in 80% yield.