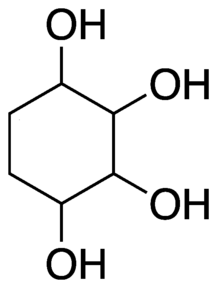
Cyclitol
In organic chemistry, a cyclitol is a cycloalkane containing at least three hydroxyl, each attached to a different ring carbon atom.
[1] The general formula for an unsubstituted cyclitol is CnH2n-x(OH)x or CnH2nOx where 3 ≤ x ≤ n. The name is also used for compounds that can be viewed as result of substituting various functional groups for the hydrogen atoms in such a molecule, as well as similar molecules with one or more double bonds in the ring.
[citation needed] Cyclitols and their derivatives are some of the compatible solutes which are formed in a plant as a response to salt or water stress.
Some cyclitols (e.g. quinic or shikimic acid) are parts of hydrolysable tannins.
Furthermore, the hydrogen and the hydroxyl on each carbon atom may lie in two possible arrangements relative to the local ring plane; so that each structural isomer may exist in several stereoisomers, depending on which side of the ring plane the hydroxyls are.