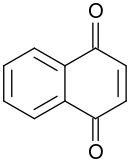
1,4-Naphthoquinone
It forms volatile yellow triclinic crystals and has a sharp odor similar to benzoquinone.
Its adduct with 1,3-butadiene can be prepared by two methods: 1) long (45 days) exposure of naphthoquinone in neat liquid butadiene taken in huge excess at room temperature in a thick-wall glass tube or 2) fast catalyzed cycloaddition at low temperature in the presence of 1 equivalent of tin(IV) chloride:[5] 1,4-Naphthoquinone is mainly used as a precursor to anthraquinone by reaction with butadiene followed by oxidation.
2-Methyl-1,4-naphthoquinone, called menadione, is a more effective coagulant than vitamin K. Other natural naphthoquinones include juglone, plumbagin, droserone.
They are cytotoxic, they have significant antibacterial, antifungal, antiviral, insecticidal, anti-inflammatory, and antipyretic properties.
Plants with naphthoquinone content are widely used in China and the countries of South America, where they are used to treat malignant and parasitic diseases.