Cinnamic acid
[5] Cinnamic acid is a central intermediate in the biosynthesis of a myriad of natural products including lignols (precursors to lignin and lignocellulose), flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids.
[citation needed] Cinnamic acid has a honey-like odor;[2] and its more volatile ethyl ester, ethyl cinnamate, is a flavor component in the essential oil of cinnamon, in which related cinnamaldehyde is the major constituent.
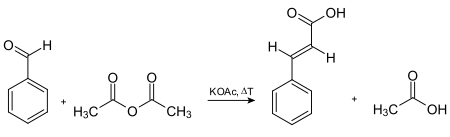
[9] The reactants for this are benzaldehyde and malonic acid in the presence of a weak base, followed by acid-catalyzed decarboxylation.
It can also be prepared by oxidation of cinnamaldehyde, condensation of benzal chloride and sodium acetate (followed by acid hydrolysis), and the Perkin reaction.
[5] Cinnamic acid can dimerize in non-polar solvents resulting in different linear free energy relationships.