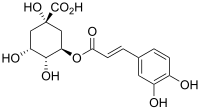
Chlorogenic acid
The hydroxylation of the coumaryl ester, i.e. installing the second hydroxy group, is catalyzed by a cytochrome P450 enzyme.
[7] Chlorogenic acid can be found in the bamboo Phyllostachys edulis,[8] as well as in many other plants,[9] such as the shoots of common heather (Calluna vulgaris).
[12] Chlorogenic acid is present in the flesh of eggplants,[13] peaches,[14] prunes[15] and coffee beans.
[22] The order of numbering of atoms on the quinic acid ring was reversed in 1976 following IUPAC guidelines, with the consequence that 3-CQA became 5-CQA, and 5-CQA became 3-CQA.
Even the 1976 IUPAC recommendations are not entirely satisfactory when applied to some of the less common chlorogenic acids.