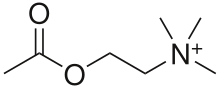
Acetylcholine receptor
Molecular biology has shown that the nicotinic and muscarinic receptors belong to distinct protein superfamilies.
Nm[1] is located in the neuromuscular junction which causes the contraction of skeletal muscles by way of end-plate potential (EPPs).
Nn causes depolarization in autonomic ganglia resulting in post ganglionic impulse.
Nicotinic receptors cause the release of catecholamine from the adrenal medulla, and also site specific excitation or inhibition in brain.
[4] In fact, relatively minor mutations, such as a change in 3 amino acids in many of these receptors can convert a cation-selective channel to an anion-selective channel gated by acetylcholine, showing that even fundamental properties can relatively easily change in evolution.
In myasthenia gravis, the receptor at the neuromuscular junction is targeted by antibodies, leading to muscle weakness.
Postsynaptic defects are the most frequent cause of CMS and often result in abnormalities in nicotinic acetylcholine receptors.
The addition of a cationic Arg into the anionic environment of the AChR binding site greatly reduces the kinetic properties of the receptor.