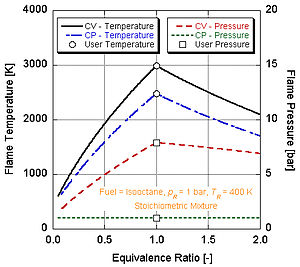
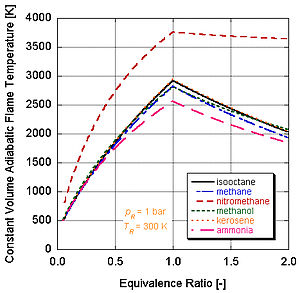
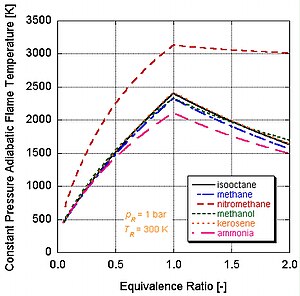
Adiabatic flame temperature
Its temperature is higher than in the constant pressure process because no energy is utilized to change the volume of the system (i.e., generate work).
In daily life, the vast majority of flames one encounters are those caused by rapid oxidation of hydrocarbons in materials such as wood, wax, fat, plastics, propane, and gasoline.
Incomplete reaction at higher temperature further curtails the effect of a larger heat of combustion.
[citation needed] Because most combustion processes that happen naturally occur in the open air, there is nothing that confines the gas to a particular volume like the cylinder in an engine.
This also assumes complete combustion (e.g. perfectly balanced, non-smoky, usually bluish flame).
Because this is a closed system, the mass of the products and reactants is the same and the first law can be written on a mass basis: We see that the adiabatic flame temperature of the constant pressure process is lower than that of the constant volume process.
If we make the assumption that combustion goes to completion (i.e. forming only CO2 and H2O), we can calculate the adiabatic flame temperature by hand either at stoichiometric conditions or lean of stoichiometry (excess air).
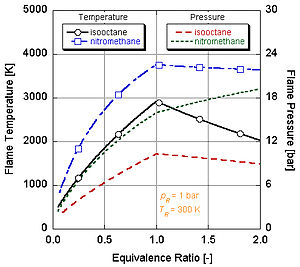
Since each molecule of nitromethane contains an oxidant with relatively high-energy bonds between nitrogen and oxygen, it can burn much hotter than hydrocarbons or oxygen-containing methanol.
However, continual running of an engine on nitromethane will eventually melt the piston and/or cylinder because of this higher temperature.
There are a number of programs available that can calculate the adiabatic flame temperature taking into account dissociation through equilibrium constants (Stanjan, NASA CEA, AFTP).
The following figure illustrates that the effects of dissociation tend to lower the adiabatic flame temperature.