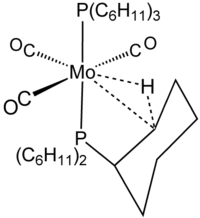
Agostic interaction
Many catalytic transformations involving oxidative addition and reductive elimination are proposed to proceed via intermediates featuring agostic interactions.
The term agostic, derived from the Ancient Greek word for "to hold close to oneself", was coined by Maurice Brookhart and Malcolm Green, on the suggestion of the classicist Jasper Griffin, to describe this and many other interactions between a transition metal and a C−H bond.
Often such agostic interactions involve alkyl or aryl groups that are held close to the metal center through an additional σ-bond.
Neutron diffraction data have shown that C−H and M┄H bond distances are 5-20% longer than expected for isolated metal hydride and hydrocarbons.
For instance, in Ziegler–Natta catalysis the highly electrophilic metal center has agostic interactions with the growing polymer chain.
The term agostic is reserved to describe two-electron, three-center bonding interactions between carbon, hydrogen, and a metal.