Aleksandr Dianin
In 1887, Dianin succeeded his father-in-law as chair of the Chemistry Department at the Imperial Medical-Surgical Academy in St. Petersburg (now the S.M.
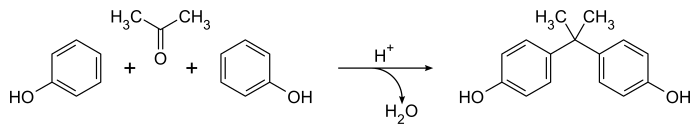
[4] The overall equation is simple, with water as the only by-product: Mechanistically, the acid catalyst converts the acetone to a carbenium ion that undergoes an electrophilic aromatic substitution reaction with the phenol, producing predominantly para-substituted products.
A second carbenium species is produced by protonation and loss of the aliphatic hydroxyl group, leading to bisphenol A (4,4'-isopropylidenediphenol) after a second aromatic substitution reaction.
[4] Catalysed dimerisation of acetone via an aldol condensation is well known, and yields diacetone alcohol and (by dehydration) mesityl oxide in both acidic[6] and basic conditions.
In cases where an olefinic moiety can interact with a phenolic hydroxyl group (typically as a result of ortho-substitution), rapid cyclisation reactions producing flavans and chromans occur.