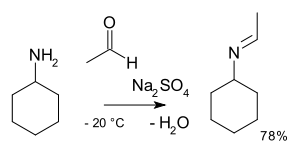
Carbonyl condensation
Primary amines react through an unstable hemiaminal intermediate which then splits off water.
Secondary amines do not lose water easily because they do not have a proton available and instead they often react further to an aminal: or when an α-carbonyl proton is present to an enamine: In acidic environment the reaction product is an iminium salt by loss of water.
Compounds containing both a primary or secondary amine and carbonyl functional group are often labile.
Aminoacetone, the simplest amino ketone, cannot be isolated as a liquid or solid,[3] and 2-aminobenzaldehyde oligomerizes in solution or in the melt.
Their equilibria strongly favor the dehydrated product, and the carbonyl is recovered only with difficulty.