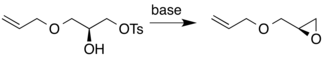
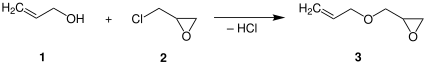Allyl glycidyl ether
[6] Radical polymerization of the propylene portion in the presence of methyl acrylate yields a block copolymer with a high epoxide content.
The alkenes can be elaborated into short polyethylene-glycol oligomers to further increase the ion-binding ability and enhance the resulting material properties.
[12] Block copolymers with ethylene oxide form micelles, which could be useful for encapsulating other molecules as part of a drug delivery system.
[14] Rather than polymerization, the alkene group can undergo a hydrosilylation reaction with siloxanes in the presence of chloroplatinic acid as catalyst.
By this reaction, allyl glycidyl ether finds use as an intermediate in the production of silane coatings for electrical applications.