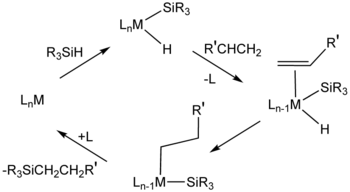
Hydrosilylation
Some cases involve insertion of alkene into M-Si bond followed by reductive elimination, the opposite of the sequence in the Chalk-Harrod mechanism.
[4] Alkynes also undergo hydrosilylation, e.g., the addition of triethylsilane to diphenylacetylene:[5] Using chiral phosphines as spectator ligands, catalysts have been developed for catalytic asymmetric hydrosilation.
A well studied reaction is the addition of trichlorosilane to styrene to give 1-phenyl-1-(trichlorosilyl)ethane: Nearly perfect enantioselectivities (ee's) can be achieved using palladium catalysts supported by binaphthyl-substituted monophosphine ligands.
[7] The resulting monolayer, which is stable and inert, inhibits oxidation of the base silicon layer, relevant to various device applications.
A peroxide-catalyzed process was reported in academic literature in 1947,[9] but the introduction of Speier's catalyst (H2PtCl6) was a big breakthrough.