Ammonium acetate
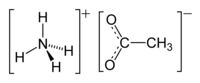
Ammonium acetate, also known as spirit of Mindererus in aqueous solution, is a chemical compound with the formula NH4CH3CO2.
Because of this, it has been used to replace cell buffers that contain non-volatile salts in preparing samples for mass spectrometry.
[8] It is also popular as a buffer for mobile phases for HPLC with ELSD and CAD-based detection for this reason.
[9] Ammonium acetate is also used as a food additive as an acidity regulator; INS number 264.
[11] Obtaining crystalline ammonium acetate is difficult on account of its hygroscopic nature.