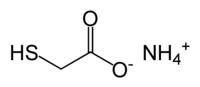
Ammonium thioglycolate
The thioglycolic acid in the perm solution reduces the disulfide cystine bonds in the cortex of the hair.
After washing, the hair is treated with a mild solution of hydrogen peroxide, which oxidizes the cysteines back to cystine.
The rigidification process is akin to the vulcanization of rubber, where commonly polysulfide linkages are used to crosslink the polymer chains.
As a result, the hair is weaker than before the permanent was applied and repeated applications over the same spot may eventually cause strand breakage.
Since polar molecules are less volatile than nonpolar ones, the glycolate substituent makes the thiol non-volatile and hence less odorous.