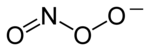
Peroxynitrite
Peroxynitrite (sometimes called peroxonitrite) is an ion with the formula ONOO−.
It is a structural isomer of nitrate, NO−3 Peroxynitrite can be prepared by the reaction of superoxide with nitric oxide:[1][2][3] It is prepared by the reaction of hydrogen peroxide with nitrite:[4] Its presence is indicated by the absorbance at 302 nm (pH 12, ε302 = 1670 M−1 cm−1).
In vivo, the concentration of carbon dioxide is about 1 mM, and its reaction with ONOO− occurs quickly.
Thus, under physiological conditions, the reaction of ONOO− with carbon dioxide to form nitrosoperoxycarbonate (ONOOCO−2) is by far the predominant pathway for ONOO−.
Approximately 66% of the time, these two radicals recombine to form carbon dioxide and nitrate.