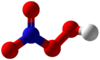
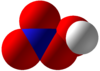
Peroxynitric acid
Peroxynitric acid or peroxonitric acid is a chemical compound with the formula HNO4.
It is an oxyacid of nitrogen, after peroxynitrous acid.
Peroxynitrate, the conjugate base of peroxynitric acid, is formed rapidly during decomposition of peroxynitrite in neutral conditions.
[4] Peroxynitric acid is formed in the atmosphere, although it is unstable, it is important as a reservoir for NO2 through the reversible radical reaction:[5]
This inorganic compound–related article is a stub.