Anaphase-promoting complex
Anaphase-promoting complex (also called the cyclosome or APC/C) is an E3 ubiquitin ligase that marks target cell cycle proteins for degradation by the 26S proteasome.
[1] It was the discovery of the APC/C (and SCF) and their key role in eukaryotic cell-cycle regulation that established the importance of ubiquitin-mediated proteolysis in cell biology.
[2][3] The APC/C's main function is to trigger the transition from metaphase to anaphase by tagging specific proteins for degradation.
Separase then triggers the cleavage of cohesin, the protein complex that binds sister chromatids together.
When securin undergoes ubiquitination by the APC/C and releases separase, which degrades cohesin, sister chromatids become free to move to opposite poles for anaphase.
These proteins target the APC/C to specific sets of substrates at different times in the cell cycle, thus driving it forward.
These two subunits catalyze ubiquitination of substrates when the C-terminal domain of Apc2 forms a tight complex with Apc11.
[1] In addition to the catalytic functionality, other core proteins of the APC are composed multiple repeat motifs with the main purpose of providing molecular scaffold support.
[8] Most notably, 4 subunits of yeast APC/C consist almost entirely of multiple repeats of the 34 amino acid tetratricopeptide residue (TPR) motif.
These TPR subunits, Cdc16,[9] Cdc27,[10] Cdc23, and Apc5, mainly provide scaffolding and support to mediate other protein-protein interactions.
Cdc27 and Cdc23 have been shown to support the binding of Cdc20 and Cdh1, as mutations in key residues of these subunits led to increased dissociation of the activators.
The subunit Apc15 plays an important role in APC/CCdc20 activation following the bi-orientation of sister chromatids across the metaphase plate.
When kinetochores are unattached to spindles, mitotic checkpoint complexes (MCC) and inhibit APC.
In the absence of Apc15, MCCs and Cdc20 remain locked on the APC/C preventing its activity once the spindle checkpoint requirements are met.
[14] One of the subunits that exhibit the TPR motif, CDC27 has been identified to interact with mitotic checkpoint proteins such as Mad2, p55CDC and BUBR1, suggesting that it may have involvement in the timing of M phase.
[15] Evidence shows that CDC27 is involved in a ternary complex with SMAD2/3 and Cdh1, which is created in response to TGFβ signalling.
A study suggests that TGF-β-induced Cdc27 phosphorylation enhances interaction between cdc27 and Cdh1–which is directly involved in activating APC.
Based upon hybrid assays in vivo and co-immunoprecipitation in vitro, it is suggested that Cdc16p, Cdc23p and Cdc27p (analogs in Sacchyromyces cerevisiae) interact and form a macromolecular complex.
In further drosophila studies, Cdk16 and cdk23 appear to be activated via phosphorylation by Polo-like kinase 1 (Plk1) and its fission yeast counterpart, appear to bind particularly to Cdc23.
Kraft et al. have shown that the substrates' D boxes bind directly to the highly conserved WD40 repeat propeller region on the APC activators.
It has been found that a Lys residue immediately C-terminal to the D box can function as a ubiquitin acceptor.
[6] Although Cdc20 and Cdh1 may serve as D and KEN box receptors, the low affinity of these co-activator–substrate interactions suggests that it is unlikely that the co-activators alone are sufficient to confer high-affinity substrate binding to the APC/CCdc20 and APC/CCdh1.
The molecular basis of the delay is unknown, but is believed to involve the key to the correct timing of anaphase initiation.
In animal cells the spindle checkpoint system contributes to the delay if it needs to correct the bi-orientation of chromosomes.
It is possible that this negative feedback is the backbone of Cdk activity controlled by M and S cyclin concentration oscillations.
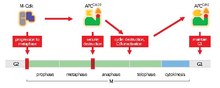
Thus, as APCCdc20 becomes inactivated during metaphase due to dephosphorylation resulting from inactive mitotic Cdks, Cdh1 is able to immediately bind to APC/C, taking Cdc20's place.
[1] Emi1 association with Cdc20 allows for the stabilization of various cyclins throughout S and G2 phase, but Emi1's removal is essential for progression through mitosis.
[1] Regulation of APC/CCdc20 activity towards metaphase substrates like securin and cyclin B may be a result of intracellular localization.