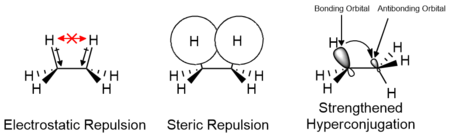Rotamer
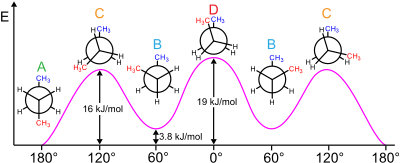
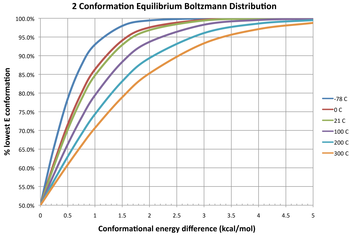
Conversely, a positive difference in free energy means the conformer already is the more stable one, so the interconversion is an unfavorable equilibrium (K < 1).
On the right side, Ek (k = 1, 2, ..., M) is the energy of conformer k, R is the molar ideal gas constant (approximately equal to 8.314 J/(mol·K) or 1.987 cal/(mol·K)), and T is the absolute temperature.
The Karplus equation relates the dihedral angle of vicinal protons to their J-coupling constants as measured by NMR.
[20] Protein side chains exhibit rotamers, whose distribution is determined by their steric interaction with different conformations of the backbone.
This effect is evident from statistical analysis of the conformations of protein side chains in the Backbone-dependent rotamer library.
Prediction of rates of many reactions involving the transition between sp2 and sp3 states, such as ketone reduction, alcohol oxidation or nucleophilic substitution is possible if all conformers and their relative stability ruled by their strain is taken into account.
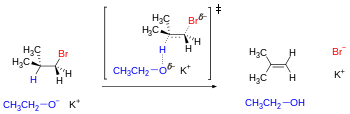
[24] One example where the rotamers become significant is elimination reactions, which involve the simultaneous removal of a proton and a leaving group from vicinal or antiperiplanar positions under the influence of a base.
For some cyclic substrates such as cyclohexane, however, an antiparallel arrangement may not be attainable depending on the substituents which might set a conformational lock.
[26] As a result, the t-Bu group "locks" the ring in the conformation where it is in the equatorial position and substitution reaction is observed.
On the other hand, cis-4-tert-butylcyclohexyl chloride undergoes elimination because antiperiplanarity of Cl and H can be achieved when the t-Bu group is in the favorable equatorial position.
The repulsion between an axial t-butyl group and hydrogen atoms in the 1,3-diaxial position is so strong that the cyclohexane ring will revert to a twisted boat conformation.
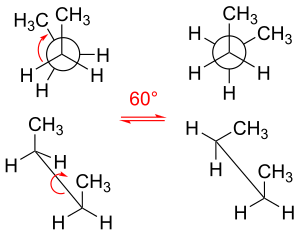
The existence of specific conformations is due to hindered rotation around sigma bonds, although a role for hyperconjugation is proposed by a competing theory.
The determination of stable conformations has also played a large role in the establishment of the concept of asymmetric induction and the ability to predict the stereochemistry of reactions controlled by steric effects.
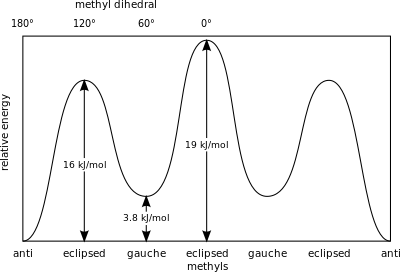
The interaction between the two methyl groups is repulsive (van der Waals strain), and an energy barrier results.
One alternative to the steric hindrance explanation is based on hyperconjugation as analyzed within the Natural Bond Orbital framework.
On the other hand, an analysis within quantitative molecular orbital theory shows that 2-orbital-4-electron (steric) repulsions are dominant over hyperconjugation.
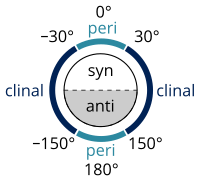
[33] Naming alkanes per standards listed in the IUPAC Gold Book is done according to the Klyne–Prelog system for specifying angles (called either torsional or dihedral angles) between substituents around a single bond:[27] Torsional strain or "Pitzer strain" refers to resistance to twisting about a bond.
Evidence for the helix structure in the crystalline state is derived from X-ray crystallography and from NMR spectroscopy and circular dichroism in solution.