Antiaromaticity
To avoid the instability of antiaromaticity, molecules may change shape, becoming non-planar and therefore breaking some of the π interactions.
[2] Cyclooctatetraene is an example of a molecule adopting a non-planar geometry to avoid the destabilization that results from antiaromaticity.
[2] The term 'antiaromaticity' was first proposed by Ronald Breslow in 1967 as "a situation in which a cyclic delocalisation of electrons is destabilising".
Non-aromatic molecules are either noncyclic, nonplanar, or do not have a complete conjugated π system within the ring.
An antiaromatic compound may also be recognized thermodynamically by measuring the energy of the cyclic conjugated π electron system.
If an experimentally determined structure of the molecule in question does not exist, a computational analysis must be performed.
The potential energy of the molecule should be probed for various geometries in order to assess any distortion from a symmetric planar conformation.
[6] This procedure is recommended because there have been multiple instances in the past where molecules which appear to be antiaromatic on paper turn out to be not truly so in actuality.
[2] The paramagnetic ring current resulting from the electron delocalization in antiaromatic compounds can be observed by NMR.
because the molecule typically loses either its planar nature or its conjugated system of π-electrons and becomes nonaromatic.
Its structure has been studied computationally via ab initio and density functional theory calculations and is confirmed to be antiaromatic.
Cyclobutadiene, for example, rapidly dimerizes with no potential energy barrier via a 2 + 2 cycloaddition reaction to form tricyclooctadiene.
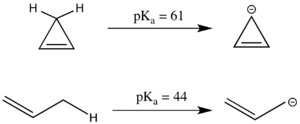
The linear compound propene has a pKa of 44, which is relatively acidic for an sp3 carbon center because the resultant allyl anion can be resonance stabilized.
The analogous cyclic system appears to have even more resonance stabilized, as the negative charge can be delocalized across three carbons instead of two.
However, the cyclopropenyl anion has 4 π electrons in a cyclic system and in fact has a substantially higher pKa than 1-propene because it is antiaromatic and thus destabilized.
For example, the aromatic species 1 can be reduced to 2 with a relatively small penalty for forming an antiaromatic system.