Antimony tribromide
Alternatively, it can be prepared by the action of bromine on a mixture of antimony sulfide and antimony trioxide at 250 °C.
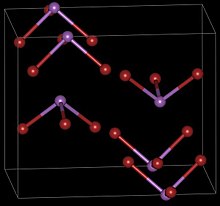
Antimony tribromide has two crystalline forms, both having orthorhombic symmetries.
When a warm carbon disulfide solution of SbBr3 is rapidly cooled, it crystallizes into the needle-like α-SbBr3, which then slowly converts to the more stable β form.
[2] Antimony tribromide hydrolyzes in water to form hydrobromic acid and antimony trioxide: It can be added to polymers such as polyethylene as a fire retardant.
[3] It is also used in the production of other antimony compounds, in chemical analysis, as a mordant, and in dyeing.