Aortic aneurysm
[1] Typically, there are no symptoms except when the aneurysm dissects or ruptures, which causes sudden, severe pain in the abdomen and lower back.
Risk factors include cigarette smoking, extreme alcoholism, advanced age, dyslipidemia, hypertension, and coronary artery disease.
In people presenting with aneurysm of the arch of the aorta, a common sign is a hoarse voice from stretching of the left recurrent laryngeal nerve, a branch of the vagus nerve that winds around the aortic arch to supply the muscles of the larynx.
[8] Risk factors for AAA include the male gender, aging, a history of smoking, hypercholesterolemia, and hypertension.
[13] Men are about four times more likely to have AA compared to women at any age, with death occurring in about 55-64% of people having AAA rupture.
Once an aneurysm has ruptured, it presents with classic symptoms of abdominal pain which is severe, constant, and radiating to the back.
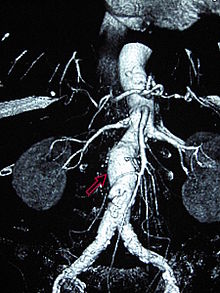
A contrast-enhanced abdominal CT scan is the best test to diagnose an AAA and guide treatment options.
[14] In 2019, some 170,000 people worldwide died from AAA rupture, with aging, smoking, and hypertension as principal factors.
Aortic aneurysm development and progression have been directly associated with a deficiency of elastin as well as a loss of collagen type 1.
[20] The risk of aneurysm enlargement may be diminished with attention to the patient's blood pressure, smoking and cholesterol levels.
[24] Screening for an aortic aneurysm so that it may be detected and treated prior to rupture is the best way to reduce the overall mortality of the disease.
Noting the results of several large, population-based screening trials, the US Centers for Medicare and Medicaid Services (CMS) now provides payment for one ultrasound study in all smokers aged 65 years or older ("SAAAVE Act").
Slowly expanding aortic aneurysms may be followed by routine diagnostic testing (i.e.: CT scan or ultrasound imaging).
However, recent data on patients aged 60–76 suggest medical management for abdominal aneurysms with a diameter of less than 5.5 cm (2 in).
[28] A 2012 Cochrane systematic review noted that further research regarding the effectiveness of CFSD for preventing a spinal cord injury is required.
[29] A 2023 systematic review suggested that rates of postoperative spinal cord ischaemia can be kept at low levels after open repair of thoracoabdominal aneurysm with the adequate precautions and perioperative manoeuvres.
[30] The majority of the surgeons believe prophylactic lumbar drains are effective in reducing spinal cord ischaemia.
[31] Neuromonitoring with motor-evoked potentials (MEP) can provide the surgeon objective criteria to direct selective intercostal reconstruction or other protective anaesthetic and surgical manoeuvres.
[30] Endovascular treatment of aortic aneurysms is a minimally invasive alternative to open surgery repair.
As compared to open surgery, EVAR has a lower risk of death in the short term and a shorter hospital stay but may not always be an option.