Aromatization
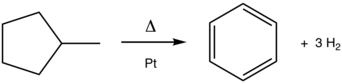
Typically aromatization is achieved by dehydrogenation of existing cyclic compounds, illustrated by the conversion of cyclohexane into benzene.
The process, which is catalyzed by platinum supported by aluminium oxide, is exemplified in the conversion methylcyclohexane (a naphthene) into toluene (an aromatic).
Platinum-catalyzed dehydrogenations of cyclohexanes and related feedstocks are the largest scale applications of this reaction (see above).
[6] Although practiced on a very small scale compared to the petrochemical routes, diverse methods have been developed for fine chemical syntheses.
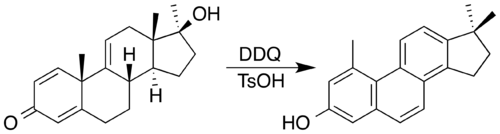
DDQ and an acid catalyst has been used to synthesise a steroid with a phenanthrene core by oxidation accompanied by a double methyl migration.
The aromatization of acyclic precursors is rarer in organic synthesis, although it is a significant component of the BTX production in refineries.
The Bergman cyclization converts an enediyne to a dehydrobenzene intermediate diradical, which abstracts hydrogen to aromatize.
Cyclodeca-3-en-1,5-diyne reacts with 1,3-cyclohexadiene to produce benzene and tetralin at 37 °C, the reaction being highly favorable owing to the formation of two new aromatic rings: