Atomic carbon
In addition, it may be considered to be the monomer of all (condensed) carbon allotropes like graphite and diamond.
Along with monocarbon, this name does distinguish the titular compound as they derived using structural information about the molecule.
Transitions between these three states are formally forbidden from occurring due to the requirement of spin flipping and or electron pairing.
This means that atomic carbon phosphoresces in the near-infrared region of the electromagnetic spectrum at 981.1 nm.
The different states of atomic carbon exhibits varying chemical behaviours.
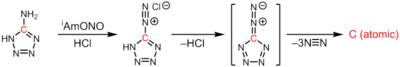
One method of synthesis, developed by Phil Shevlin has done the principal work in the field., is by passing a large current through two adjacent carbon rods, generating an electric arc.
A clean source of atomic carbon can be obtained based on the thermal decomposition of tantalum carbide.
Normally, a sample of atomic carbon exists as a mixture of excited states in addition to the ground-state in thermodynamic equilibrium.
As atomic carbon is an electron-deficient species, it spontaneously autopolymerises in its pure form, or converts to an adduct upon treatment with a Lewis acid or base.
Typical reactions with organic compounds include:[5] With water insertion into the O-H bond forms the carbene, H-C-OH that rearranges to formaldehyde, HCHO.