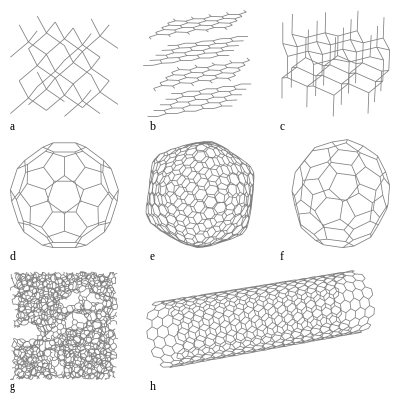
Allotropes of carbon
In recent decades, many more allotropes have been discovered and researched, including ball shapes such as buckminsterfullerene and sheets such as graphene.
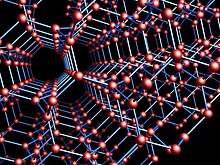
These tetrahedrons together form a 3-dimensional network of six-membered carbon rings in the chair conformation, allowing for zero bond angle strain.
Diamonds are embedded in drill tips and saw blades or ground into a powder for use in grinding and polishing applications (due to its extraordinary hardness).
This helps explain why 80% of mined diamonds (equal to about 100 million carats or 20 tonnes annually) are unsuitable for use as gemstones and known as bort, are destined for industrial use.
With the continuing advances being made in the production of synthetic diamond, future applications are beginning to become feasible.
[citation needed] Graphite, named by Abraham Gottlob Werner in 1789, from the Greek γράφειν (graphein, "to draw/write", for its use in pencils) is one of the most common allotropes of carbon.
Graphite conducts electricity, due to delocalization of the pi bond electrons above and below the planes of the carbon atoms.
Although it might be thought that this industrially important property is due entirely to the loose interlamellar coupling between sheets in the structure, in fact in a vacuum environment (such as in technologies for use in space), graphite was found to be a very poor lubricant.
When a large number of crystallographic defects (physical) bind these planes together, graphite loses its lubrication properties and becomes pyrolytic carbon, a useful material in blood-contacting implants such as prosthetic heart valves.
[citation needed] Natural and crystalline graphites are not often used in pure form as structural materials due to their shear-planes, brittleness and inconsistent mechanical properties.
In its pure glassy (isotropic) synthetic forms, pyrolytic graphite and carbon fiber graphite are extremely strong, heat-resistant (to 3000 °C) materials, used in reentry shields for missile nosecones, solid rocket engines, high temperature reactors, brake shoes and electric motor brushes.
A single layer of graphite is called graphene and has extraordinary electrical, thermal, and physical properties.
It can be produced by epitaxy on an insulating or conducting substrate or by mechanical exfoliation (repeated peeling) from graphite.
Lonsdaleite is an allotrope sometimes called "hexagonal diamond", formed from graphite present in meteorites upon their impact on the earth.
"Hexagonal diamond" has also been synthesized in the laboratory, by compressing and heating graphite either in a static press or using explosives.
[10][11][12] Graphenylene[13] is a single layer carbon material with biphenylene-like subunits as basis in its hexagonal lattice structure.
However, they are products of pyrolysis (the process of decomposing a substance by the action of heat), which does not produce true amorphous carbon under normal conditions.
As of the early twenty-first century, the chemical and physical properties of fullerenes are still under heavy study, in both pure and applied research labs.
Their name is derived from their size, since the diameter of a nanotube is on the order of a few nanometers (approximately 50,000 times smaller than the width of a human hair), while they can be up to several centimeters in length.
The geometric topology of the structure is determined by the presence of ring defects, such as heptagons and octagons, to graphene's hexagonal lattice.
The name, ZTC, derives from their origin inside the pores of zeolites, crystalline silicon dioxide minerals.
A vapor of carbon-containing molecules is injected into the zeolite, where the carbon gathers on the pores' walls, creating the negative curve.
A team generated structures by decorating the pores of a zeolite with carbon through a Monte Carlo method.
The preparation of glassy carbon involves subjecting the organic precursors to a series of heat treatments at temperatures up to 3000 °C.
Unlike many non-graphitizing carbons, they are impermeable to gases and are chemically extremely inert, especially those prepared at very high temperatures.
Thus, while normal graphite is reduced to a powder by a mixture of concentrated sulfuric and nitric acids at room temperature, glassy carbon is unaffected by such treatment, even after several months.
Each cluster is about 6 nanometers wide and consists of about 4000 carbon atoms linked in graphite-like sheets that are given negative curvature by the inclusion of heptagons among the regular hexagonal pattern.
These structures exhibit high porosity and specific surface areas, with highly tunable pore diameters, making them promising materials for supercapacitor-based energy storage, water filtration and capacitive desalinization, catalyst support, and cytokine removal.
The system of carbon allotropes spans an astounding range of extremes, considering that they are all merely structural formations of the same element.