Atom
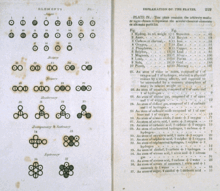
[4] In the early 1800s, John Dalton compiled experimental data gathered by him and other scientists and discovered a pattern now known as the "law of multiple proportions".
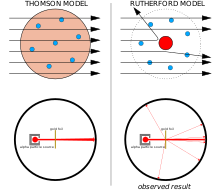
This shouldn't have been possible according to the Thomson model of the atom, whose charges were too diffuse to produce a sufficiently strong electric field.
Only such an intense concentration of charge, anchored by its high mass, could produce an electric field that could deflect the alpha particles so strongly.
Circular motion counts as acceleration, which means that an electron orbiting a central charge should spiral down into that nucleus as it loses speed.
[20] This quantization was used to explain why the electrons' orbits are stable and why elements absorb and emit electromagnetic radiation in discrete spectra.
In 1928, Walter Bothe observed that beryllium emitted a highly penetrating, electrically neutral radiation when bombarded with alpha particles.
In 1932, Chadwick exposed various elements, such as hydrogen and nitrogen, to the mysterious "beryllium radiation", and by measuring the energies of the recoiling charged particles, he deduced that the radiation was actually composed of electrically neutral particles which could not be massless like the gamma ray, but instead were required to have a mass similar to that of a proton.
The electron is the least massive of these particles by four orders of magnitude at 9.11×10−31 kg, with a negative electrical charge and a size that is too small to be measured using available techniques.
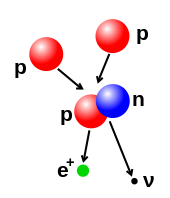
Nuclear fusion occurs when multiple atomic particles join to form a heavier nucleus, such as through the energetic collision of two nuclei.
For example, at the core of the Sun protons require energies of 3 to 10 keV to overcome their mutual repulsion—the coulomb barrier—and fuse together into a single nucleus.
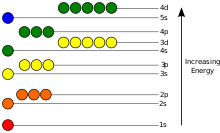
This behavior is defined by an atomic orbital, a mathematical function that characterises the probability that an electron appears to be at a particular location when its position is measured.
[51] Only a discrete (or quantized) set of these orbitals exist around the nucleus, as other possible wave patterns rapidly decay into a more stable form.
An additional 35 radioactive nuclides have half-lives longer than 100 million years, and are long-lived enough to have been present since the birth of the Solar System.
The deformation depends on the field magnitude and the orbital type of outer shell electrons, as shown by group-theoretical considerations.
Aspherical deviations might be elicited for instance in crystals, where large crystal-electrical fields may occur at low-symmetry lattice sites.
[79] Every element has one or more isotopes that have unstable nuclei that are subject to radioactive decay, causing the nucleus to emit particles or electromagnetic radiation.
A few large nuclei explode into two or more charged fragments of varying masses plus several neutrons, in a decay called spontaneous nuclear fission.
This is analogous to the angular momentum of an object that is spinning around its center of mass, although strictly speaking these particles are believed to be point-like and cannot be said to be rotating.
The orbitals of neighboring atoms overlap and a lower energy state is achieved when the spins of unpaired electrons are aligned with each other, a spontaneous process known as an exchange interaction.
Spectroscopic measurements of the strength and width of atomic spectral lines allow the composition and physical properties of a substance to be determined.
[92] When an atom is in an external magnetic field, spectral lines become split into three or more components; a phenomenon called the Zeeman effect.
The interaction of the magnetic field with the atom shifts these electron configurations to slightly different energy levels, resulting in multiple spectral lines.
[93] The presence of an external electric field can cause a comparable splitting and shifting of spectral lines by modifying the electron energy levels, a phenomenon called the Stark effect.
This physical property is used to make lasers, which can emit a coherent beam of light energy in a narrow frequency band.
[106][107] Because of the distances involved, both electrodes need to be extremely stable; only then periodicities can be observed that correspond to individual atoms.
Where the temperature is much higher than ionization potential, the matter exists in the form of plasma—a gas of positively charged ions (possibly, bare nuclei) and electrons.
[125] Natural deposits of plutonium and neptunium are produced by neutron capture in uranium ore.[134] The Earth contains approximately 1.33×1050 atoms.
[135] Although small numbers of independent atoms of noble gases exist, such as argon, neon, and helium, 99% of the atmosphere is bound in the form of molecules, including carbon dioxide and diatomic oxygen and nitrogen.
At the surface of the Earth, an overwhelming majority of atoms combine to form various compounds, including water, salt, silicates, and oxides.
Atoms can also combine to create materials that do not consist of discrete molecules, including crystals and liquid or solid metals.