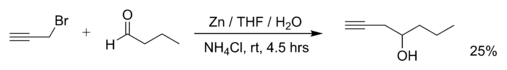
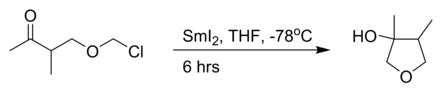
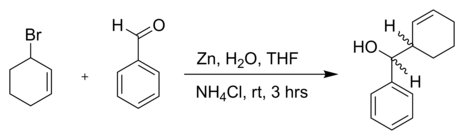
Barbier reaction
The reaction can be performed using magnesium, aluminium, zinc, indium, tin, samarium, barium or their salts.
The reaction product is a primary, secondary or tertiary alcohol.
[1] Unlike many Grignard reagents, the organometallic species generated in a Barbier reaction are unstable and thus cannot be stored or sold commercially.
Barbier reactions are nucleophilic addition reactions that involve relatively inexpensive, water insensitive metals (e.g zinc powder) or metal compounds.
For this reason, it is possible in many cases to run the reaction in water, making the procedure part of green chemistry.