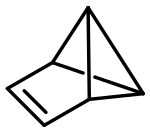
Benzvalene
[1] It was first synthesized in 1967 by K. E. Wilzbach et al. [2] via photolysis of benzene and the synthesis was later improved by Thomas J. Katz et al.[3][4] The 1971 synthesis consisted of treating cyclopentadiene with methyllithium in dimethyl ether and then with dichloromethane and methyllithium in diethyl ether at −45 °C.
Due to the high steric strain present in benzvalene, the pure compound (~71 kcal/mol higher in energy than benzene) easily detonates, for example by scratching.
The compound converts to benzene with a chemical half-life of approximately 10 days.
[7] This polymer contains highly strained bicyclobutane rings which again makes it a sensitive material.
The rings can be isomerized to 1,3-dienes and for this reason polybenzvalene has been investigated as a precursor to polyacetylene.