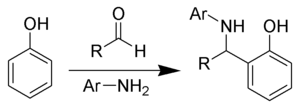
Betti reaction
Betti worked at many universities in Italy, including Florence, Cagliari, Siena, Genoa and Bologna, where he was the successor of Giacomo Ciamician.
Betti's main research was focused on stereochemistry, and the resolution of racemic compounds, the relationship between molecular constitution and optical rotation, as well asymmetric synthesis using chiral auxiliaries or in the presence of polarized light.
[1] Today, the name has grown to refer to any reaction of aldehydes, primary aromatic amines and phenols producing α-aminobenzylphenols.
The carbonyl is then reformed and a double bond in the benzene ring attacks the carbon atom in the pronated imine cation.
The oxygen, which now has a negative formal charge, then attacks a hydrogen on the hydronium, resulting in an α-aminobenzylphenol, with water as the only byproduct.