Cadmium sulfate
Cadmium sulfate is the name of a series of related inorganic compounds with the formula CdSO4·xH2O.
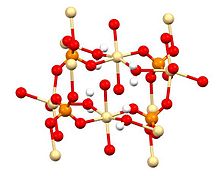
X-ray crystallography shows that CdSO4·H2O is a typical coordination polymer.
[5] Cadmium sulfate hydrate can be prepared by the reaction of cadmium metal or its oxide or hydroxide with dilute sulfuric acid: The anhydrous material can be prepared using sodium persulfate:[citation needed] Cadmium sulfates occur as the following rare minerals drobecite (CdSO4·4H2O), voudourisite (monohydrate), and lazaridisite (the 8/3-hydrate).
It is also a precursor to cadmium-based pigment such as cadmium sulfide.
It is also used for electrolyte in a Weston standard cell as well as a pigment in fluorescent screens.