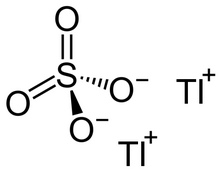
Thallium(I) sulfate
It is a precursor to thallium(I) sulfide (Tl2S), which exhibits high electrical conductivity when exposed to infrared light.
In aqueous solution, the thallium(I) cations and the sulfate anions are separated and highly solvated.
Since thallium(I) sulfate is a simple powder with indistinctive properties, it can easily be mistaken for more innocuous chemicals.
Due to its poisonous nature, many western countries have banned the use of thallium(I) sulfate in products for home use and many companies have also stopped using this compound.
Thallium(I) sulfate was used in Israel to control the rodent population; it is suspected that in the 1950s, this resulted in the disappearance of the brown fish owl.