Mercury(I) sulfate
[3] Mercury(I) sulfate is a metallic compound that is a white, pale yellow or beige powder.
[4] It is a metallic salt of sulfuric acid formed by replacing both hydrogen atoms with mercury(I).
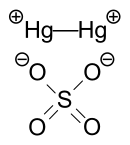
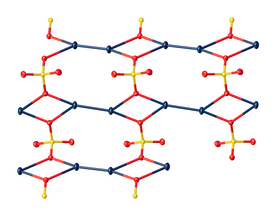
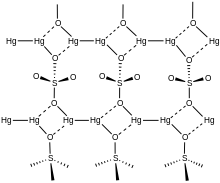
In the crystal, mercurous sulfate is made up of Hg22+ center with an Hg-Hg distance of about 2.50 Å.
The SO42− anions form both long and short Hg-O bonds ranging from 2.23 to 2.93 Å.
It has a rather low solubility (about one gram per liter); diffusion from the cathode system is not excessive; and it is sufficient to give a large potential at a mercury electrode.