Calcium hexaboride
It is an important material due to its high electrical conductivity [disputed – discuss], hardness, chemical stability, and melting point.
CaB6 has been investigated in the past due to a variety of peculiar physical properties, such as superconductivity, valence fluctuation and Kondo effects.
[4] CaB6 is insoluble in H2O, MeOH (methanol), and EtOH (ethanol) and dissolves slowly in acids.
The low, semi-metallic conductivity of many CaB6 samples can be explained by unintentional doping due to impurities and possible non-stoichiometry.
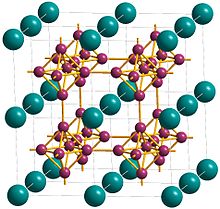
[8] The crystal structure of calcium hexaboride is a cubic lattice with calcium at the cell centre and compact, regular octahedra of boron atoms linked at the vertices by B-B bonds to give a three-dimensional boron network.
[12] Calcium hexaboride is used in the manufacturing of boron-alloyed steel[5] and as a deoxidation agent in production of oxygen-free copper.
When used at elevated temperature, calcium hexaboride will oxidize degrading its properties and shortening its usable lifespan.