Biology of depression
Scientific studies have found that different brain areas show altered activity in humans with major depressive disorder (MDD).
[14] A 2003 study proposed that a gene-environment interaction (GxE) may explain why life stress is a predictor for depressive episodes in some individuals, but not in others, depending on an allelic variation of the serotonin-transporter-linked promoter region (5-HTTLPR).
[16] BDNF polymorphisms have also been hypothesized to have a genetic influence, but early findings and research failed to replicate in larger samples, and the effect sizes found by earlier estimates are inconsistent with the observed polygenicity of depression.
[16] A 2015 GWAS study in Han Chinese women positively identified two variants in intronic regions near SIRT1 and LHPP with a genome-wide significant association.
[20] A meta analysis of 182 case controlled genetic studies published in 2008 found Apolipoprotein E epsilon 2 to be protective, and GNB3 825T, MTHFR 677T, SLC6A4 44bp insertion or deletions, and SLC6A3 40 bpVNTR 9/10 genotype to confer risk.
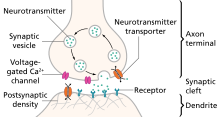
Prolonged wakefulness due to sleep deprivation[22] activates serotonergic neurons, leading to processes similar to the therapeutic effect of antidepressants, such as the selective serotonin reuptake inhibitors (SSRIs).
SSRIs may directly depend on the increase of central serotonergic neurotransmission for their therapeutic effect, the same system that impacts cycles of sleep and wakefulness.
Normal serotonin levels have been linked to mood and behaviour regulation, sleep, and digestion; norepinephrine to the fight-or-flight response; and dopamine to movement, pleasure, and motivation.
Some have also proposed the relationship between monoamines and phenotypes such as serotonin in sleep and suicide, norepinephrine in dysphoria, fatigue, apathy, cognitive dysfunction, and dopamine in loss of motivation and psychomotor symptoms.
One explanation for this therapeutic lag is that the initial increase in synaptic serotonin is only temporary, as firing of serotonergic neurons in the dorsal raphe adapt via the activity of 5-HT1A autoreceptors.
The therapeutic effect of antidepressants is thought to arise from autoreceptor desensitization over a period of time, eventually elevating firing of serotonergic neurons.
People with MDD have an increased reward response to dextroamphetamine compared to controls, and it has been suggested that this results from hypersensitivity of dopaminergic pathways due to natural hypoactivity.
[52] As of 2012, efforts to determine differences in neurotransmitter receptor expression or for function in the brains of people with MDD using positron emission tomography (PET) had shown inconsistent results.
Proponents of the monoamine hypothesis argue that this lag is that the neurotransmitter activity enhancement is the result of auto receptor desensitization, which can take weeks.
[69] Another meta analysis reported elevated hippocampus and thalamus activity in a subgroup of depressed subjects who were medication naive, not elderly, and had no comorbidities.
[73] One meta analysis of functional neuroimaging in depression observed a pattern of abnormal neural activity hypothesized to reflect an emotional processing bias.
The ACC was divided into pregenual (pgACC) and subgenual regions (sgACC), with the former being electrophysiologically associated with fear, and the latter being metabolically implicated in sadness in healthy subjects.
Anhedonia is broadly defined as a reduced ability to feel pleasure, but questionnaires and clinical assessments rarely distinguish between motivational "wanting" and consummatory "liking".
General affective blunting may explain "anhedonic" symptoms in depression, as meta analysis of both positive and negative stimuli reveal reduced rating of intensity.
Residual anhedonia that is not well targeted by serotonergic antidepressants is hypothesized to result from inhibition of dopamine release by activation of 5-HT2C receptors in the striatum.
[102][103] Studies of resting state activity have utilized a number of indicators of resting state activity, including regional homogeneity (ReHO), amplitude of low frequency fluctuations (ALFF), fractional amplitude of low frequency fluctuations (fALFF), arterial spin labeling (ASL), and positron emission tomography (PET) measures of regional cerebral blood flow or metabolism.
This region is extremely rich in serotonin transporters and is considered as a governor for a vast network involving areas like hypothalamus and brain stem, which influences changes in appetite and sleep; the amygdala and insula, which affect the mood and anxiety; the hippocampus, which plays an important role in memory formation; and some parts of the frontal cortex responsible for self-esteem.
[116][117] Stress can cause depression and depression-like symptoms through monoaminergic changes in several key brain regions as well as suppression in hippocampal neurogenesis.
Anhedonia and motivational deficits may, for example, be assessed via examining an animal's level of engagement with rewarding stimuli such as sucrose or intracranial self-stimulation.
In animal models of depression, elevated activity has been reported in LHb neurons that project to the ventral tegmental area (ostensibly reducing dopamine release).
Excitation of glutaminergic inputs from the ventral hippocampus reduces social interactions, and enhancing these projections produces susceptibility to stress-induced depression.
[150] A meta analysis published in 2014 found the use of anti-inflammatory drugs such as NSAIDs and investigational cytokine inhibitors reduced depressive symptoms.
[158] These markers include high levels of RNS and ROS which have been shown to influence chronic inflammation, damaging the electron transport chain and biochemical cascades in mitochondria.
[162][163] This network is involved in high level cognitive functions such as maintaining and using information in working memory, problem solving, and decision making.
[161] Individuals who have a tendency to experience negative emotional states (scoring high on measures of neuroticism) show an increase in the right anterior insula during decision-making, even if the decision has already been made.