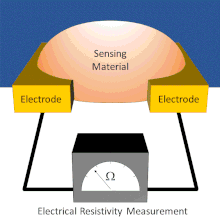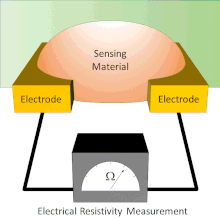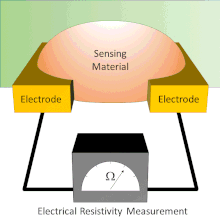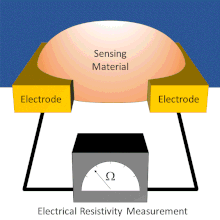
Chemiresistor
Several different materials have chemiresistor properties: semiconducting metal oxides, some conductive polymers,[3] and nanomaterials like graphene, carbon nanotubes and nanoparticles.
As far back as 1965 there are reports of semiconductor materials exhibiting electrical conductivities that are strongly affected by ambient gases and vapours.
[7] The chemiresistive material they investigated was copper phthalocyanine, and they demonstrated that its resistivity decreased in the presence of ammonia vapour at room temperature.
[2][8][9] The ability of chemiresistors to provide accurate real-time information about the environment through small devices that require minimal electricity makes them an appealing addition to the internet of things.
Electrodes are typically made of conductive metals such as gold and chromium which make good ohmic contact with thin films.
Sensors made from metal oxides require high temperatures (200 °C or higher) to operate because, in order for the resistivity to change, an activation energy must be overcome.
[15] It has been used in sensors to detect vapour-phase molecules,[16][17][18] pH,[19] proteins,[19] bacteria,[20] and simulated chemical warfare agents.
[29][30][31][32] It has been shown that a chemical species can alter the resistance of a bundle of single-walled carbon nanotubes through multiple mechanisms.
[41] The width of this separation defines the barrier that electrons must tunnel through when a voltage is applied and electric current flows.
As new chemical species enter the matrix it changes the inter-particle separation which in turn affects the electrical resistance.
[45][46] Analytes diffuse into the SAMs at proportions defined by their partition coefficient and this characterizes the selectivity and sensitivity of the chemiresistor material.