Chirality (chemistry)
In chemistry, a molecule or ion is called chiral (/ˈkaɪrəl/) if it cannot be superposed on its mirror image by any combination of rotations, translations, and some conformational changes.
[1][2][3][4] The terms are derived from Ancient Greek χείρ (cheir) 'hand'; which is the canonical example of an object with this property.
A chiral molecule or ion exists in two stereoisomers that are mirror images of each other,[5] called enantiomers; they are often distinguished as either "right-handed" or "left-handed" by their absolute configuration or some other criterion.
In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct (different) groups attached to it in a tetrahedral geometry.
Less commonly, other atoms like N, P, S, and Si can also serve as stereocenters, provided they have four distinct substituents (including lone pair electrons) attached to them.
For example, despite having chiral gauche conformers that belong to the C2 point group, butane is considered achiral at room temperature because rotation about the central C–C bond rapidly interconverts the enantiomers (3.4 kcal/mol barrier).
However, if the temperature in question is low enough, the process that interconverts the enantiomeric chiral conformations becomes slow compared to a given timescale.
The relevant timescale is, to some degree, arbitrarily defined: 1000 seconds is sometimes employed, as this is regarded as the lower limit for the amount of time required for chemical or chromatographic separation of enantiomers in a practical sense.
A chiral compound can contain no improper axis of rotation (Sn), which includes planes of symmetry and inversion center.
In order to have a mirror plane, the cyclohexane ring would have to be flat, widening the bond angles and giving the conformation a very high energy.
This stereogenic center usually has four or more bonds to different groups, and may be carbon (as in many biological molecules), phosphorus (as in many organophosphates), silicon, or a metal (as in many chiral coordination compounds).
This is the case, for example, of most amines with three different substituents (NRR′R″), because of the low energy barrier for nitrogen inversion.
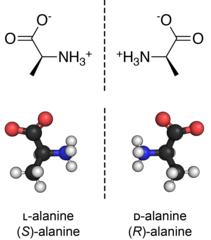
Chirality is an intrinsic part of the identity of a molecule, so the systematic name includes details of the absolute configuration (R/S, D/L, or other designations).
Many biologically active molecules are chiral, including the naturally occurring amino acids (the building blocks of proteins) and sugars.
dextro- and levo-rotation (the clockwise and counterclockwise optical rotation of plane-polarized light) uses similar notation, but shouldn't be confused.
In a solution, the (−)-form, or levorotatory form, of an optical isomer rotates the plane of a beam of linearly polarized light counterclockwise.
Louis Pasteur used this method to separate left-handed and right-handed sodium ammonium tartrate crystals in 1849.
Liquid chromatography (HPLC and TLC) may also be used as an analytical method for the direct separation of enantiomers and the control of enantiomeric purity, e.g. active pharmaceutical ingredients (APIs) which are chiral.
[19][20] The rotation of plane polarized light by chiral substances was first observed by Jean-Baptiste Biot in 1812,[25] and gained considerable importance in the sugar industry, analytical chemistry, and pharmaceuticals.
[29] At one time, chirality was thought to be restricted to organic chemistry, but this misconception was overthrown by the resolution of a purely inorganic compound, a cobalt complex called hexol, by Alfred Werner in 1911.